| Identification |
|---|
| Name | Dihydrolipoyl dehydrogenase, mitochondrial |
|---|
| Synonyms | - Dihydrolipoamide dehydrogenase
- Glycine decarboxylase complex subunit L
- Lipoamide dehydrogenase component of pyruvate dehydrogenase complex
- Pyruvate dehydrogenase complex E3 component
|
|---|
| Gene Name | LPD1 |
|---|
| Enzyme Class | |
|---|
| Biological Properties |
|---|
| General Function | Involved in oxidoreductase activity |
|---|
| Specific Function | Lipoamide dehydrogenase is a component of the alpha- ketoacid dehydrogenase complexes. This includes the pyruvate dehydrogenase complex, which catalyzes the overall conversion of pyruvate to acetyl-CoA and CO(2). Acts also as component of the glycine cleavage system (glycine decarboxylase complex), which catalyzes the degradation of glycine |
|---|
| Cellular Location | Mitochondrion matrix |
|---|
| SMPDB Pathways | |
|---|
| KEGG Pathways | | Citrate cycle (TCA cycle) | ec00020 | 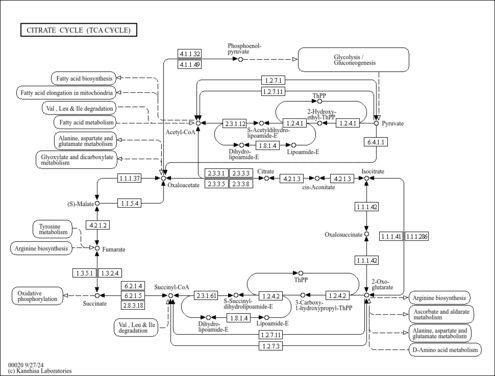 | | Glycine, serine and threonine metabolism | ec00260 |  | | Glycolysis / Gluconeogenesis | ec00010 | 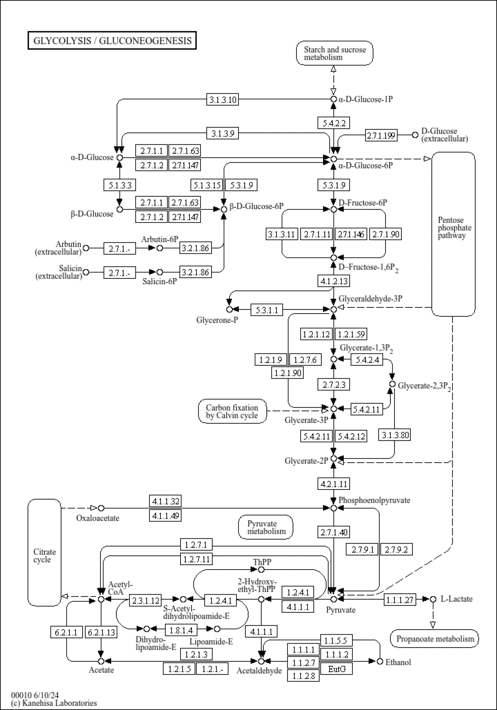 | | Pyruvate metabolism | ec00620 | 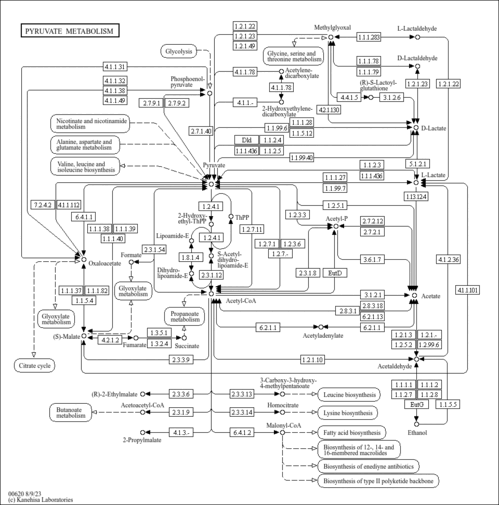 | | Valine, leucine and isoleucine degradation | ec00280 |  |
|
|---|
| SMPDB Reactions | Not Available |
|---|
| KEGG Reactions | Not Available |
|---|
| Metabolites | | YMDB ID | Name | View |
|---|
| YMDB00016 | Glycine | Show | | YMDB00045 | Coenzyme A | Show | | YMDB00110 | NAD | Show | | YMDB00143 | NADH | Show | | YMDB00153 | Oxoglutaric acid | Show | | YMDB00175 | Pyruvic acid | Show | | YMDB00312 | Acetyl-CoA | Show | | YMDB00412 | NAD(+) | Show | | YMDB00423 | Ammonium | Show | | YMDB00483 | 5,6,7,8-Tetrahydrofolic acid | Show | | YMDB00514 | Succinyl-CoA | Show | | YMDB00696 | S(8)-aminomethyldihydrolipoamide | Show | | YMDB00697 | lipoamide | Show | | YMDB00747 | S(8)-succinyldihydrolipoamide | Show | | YMDB00848 | (6R)-5,10-methylenetetrahydrofolic acid | Show | | YMDB00862 | hydron | Show | | YMDB00899 | Dihydrolipoamide | Show | | YMDB00912 | Carbon dioxide | Show |
|
|---|
| GO Classification | | Component |
|---|
| cell part | | intracellular part | | cytoplasm | | Function |
|---|
| oxidoreductase activity, acting on NADH or NADPH | | oxidoreductase activity, acting on a sulfur group of donors, NAD or NADP as acceptor | | dihydrolipoyl dehydrogenase activity | | catalytic activity | | binding | | nucleoside binding | | purine nucleoside binding | | adenyl nucleotide binding | | FAD or FADH2 binding | | oxidoreductase activity | | Process |
|---|
| oxidation reduction | | cellular process | | cellular homeostasis | | metabolic process | | cell redox homeostasis |
|
|---|
| Gene Properties |
|---|
| Chromosome Location | chromosome 6 |
|---|
| Locus | YFL018C |
|---|
| Gene Sequence | >1500 bp
ATGTTAAGAATCAGATCACTCCTAAATAATAAGCGTGCCTTTTCGTCCACAGTCAGGACA
TTGACCATTAACAAGTCACATGATGTAGTCATCATCGGTGGTGGCCCTGCTGGTTACGTG
GCTGCTATCAAAGCTGCTCAATTGGGATTTAACACTGCATGTGTAGAAAAAAGAGGCAAA
TTAGGCGGTACCTGTCTTAACGTTGGATGTATCCCCTCCAAAGCACTTCTAAATAATTCT
CATTTATTCCACCAAATGCATACGGAAGCGCAAAAGAGAGGTATTGACGTCAACGGTGAT
ATCAAAATTAACGTAGCAAACTTCCAAAAGGCTAAGGATGACGCTGTTAAGCAATTAACT
GGAGGTATTGAGCTTCTGTTCAAGAAAAATAAGGTCACCTATTATAAAGGTAATGGTTCA
TTCGAAGACGAAACGAAGATCAGAGTAACTCCCGTTGATGGGTTGGAAGGCACTGTCAAG
GAAGACCACATACTAGATGTTAAGAACATCATAGTCGCCACGGGCTCTGAAGTTACACCC
TTCCCCGGTATTGAAATAGATGAGGAAAAAATTGTCTCTTCAACAGGTGCTCTTTCGTTA
AAGGAAATTCCCAAAAGATTAACCATCATTGGTGGAGGAATCATCGGATTGGAAATGGGT
TCAGTTTACTCTAGATTAGGCTCCAAGGTTACTGTAGTAGAATTTCAACCTCAAATTGGT
GCATCTATGGACGGCGAGGTTGCCAAAGCCACCCAAAAGTTCTTGAAAAAGCAAGGTTTG
GACTTCAAATTAAGCACCAAAGTTATTTCTGCAAAGAGAAACGACGACAAGAACGTCGTC
GAAATTGTTGTAGAAGATACTAAAACGAATAAGCAAGAAAATTTGGAAGCTGAAGTTTTG
CTGGTTGCTGTTGGTAGAAGACCTTACATTGCTGGCTTAGGGGCTGAAAAGATTGGATTA
GAAGTAGACAAAAGGGGACGCCTAGTCATTGATGACCAATTTAATTCCAAGTTCCCACAC
ATTAAAGTGGTAGGAGATGTTACATTTGGTCCAATGCTGGCTCACAAAGCCGAAGAGGAA
GGTATTGCAGCTGTCGAAATGTTGAAAACTGGTCACGGTCATGTCAACTATAACAACATT
CCTTCGGTCATGTATTCTCACCCAGAAGTAGCATGGGTTGGTAAAACCGAAGAGCAATTG
AAAGAAGCCGGCATTGACTATAAAATTGGTAAGTTCCCCTTTGCGGCCAATTCAAGAGCC
AAGACCAACCAAGACACTGAAGGTTTCGTGAAGATTTTGATCGATTCCAAGACCGAGCGT
ATTTTGGGGGCTCACATTATCGGTCCAAATGCCGGTGAAATGATTGCTGAAGCTGGCTTA
GCCTTAGAATATGGCGCTTCCGCAGAAGATGTTGCTAGGGTCTGCCATGCTCATCCTACT
TTGTCCGAAGCATTTAAGGAAGCTAACATGGCTGCCTATGATAAAGCTATTCATTGTTGA
|
|---|
| Protein Properties |
|---|
| Pfam Domain Function | |
|---|
| Protein Residues | 499 |
|---|
| Protein Molecular Weight | 54009.69922 |
|---|
| Protein Theoretical pI | 8.22 |
|---|
| PDB File | show |
|---|
| Signalling Regions | |
|---|
| Transmembrane Regions | |
|---|
| Protein Sequence | >Dihydrolipoyl dehydrogenase, mitochondrial
MLRIRSLLNNKRAFSSTVRTLTINKSHDVVIIGGGPAGYVAAIKAAQLGFNTACVEKRGK
LGGTCLNVGCIPSKALLNNSHLFHQMHTEAQKRGIDVNGDIKINVANFQKAKDDAVKQLT
GGIELLFKKNKVTYYKGNGSFEDETKIRVTPVDGLEGTVKEDHILDVKNIIVATGSEVTP
FPGIEIDEEKIVSSTGALSLKEIPKRLTIIGGGIIGLEMGSVYSRLGSKVTVVEFQPQIG
ASMDGEVAKATQKFLKKQGLDFKLSTKVISAKRNDDKNVVEIVVEDTKTNKQENLEAEVL
LVAVGRRPYIAGLGAEKIGLEVDKRGRLVIDDQFNSKFPHIKVVGDVTFGPMLAHKAEEE
GIAAVEMLKTGHGHVNYNNIPSVMYSHPEVAWVGKTEEQLKEAGIDYKIGKFPFAANSRA
KTNQDTEGFVKILIDSKTERILGAHIIGPNAGEMIAEAGLALEYGASAEDVARVCHAHPT
LSEAFKEANMAAYDKAIHC |
|---|
| References |
|---|
| External Links | |
|---|
| General Reference | - Browning, K. S., Uhlinger, D. J., Reed, L. J. (1988). "Nucleotide sequence for yeast dihydrolipoamide dehydrogenase." Proc Natl Acad Sci U S A 85:1831-1834.3279419
- Ross, J., Reid, G. A., Dawes, I. W. (1988). "The nucleotide sequence of the LPD1 gene encoding lipoamide dehydrogenase in Saccharomyces cerevisiae: comparison between eukaryotic and prokaryotic sequences for related enzymes and identification of potential upstream control sites." J Gen Microbiol 134:1131-1139.3058861
- Murakami, Y., Naitou, M., Hagiwara, H., Shibata, T., Ozawa, M., Sasanuma, S., Sasanuma, M., Tsuchiya, Y., Soeda, E., Yokoyama, K., et, a. l. .. (1995). "Analysis of the nucleotide sequence of chromosome VI from Saccharomyces cerevisiae." Nat Genet 10:261-268.7670463
- Sinclair, D. A., Dawes, I. W. (1995). "Genetics of the synthesis of serine from glycine and the utilization of glycine as sole nitrogen source by Saccharomyces cerevisiae." Genetics 140:1213-1222.7498764
- Ghaemmaghami, S., Huh, W. K., Bower, K., Howson, R. W., Belle, A., Dephoure, N., O'Shea, E. K., Weissman, J. S. (2003). "Global analysis of protein expression in yeast." Nature 425:737-741.14562106
- Toyoda, T., Suzuki, K., Sekiguchi, T., Reed, L. J., Takenaka, A. (1998). "Crystal structure of eucaryotic E3, lipoamide dehydrogenase from yeast." J Biochem 123:668-674.9538259
|
|---|




