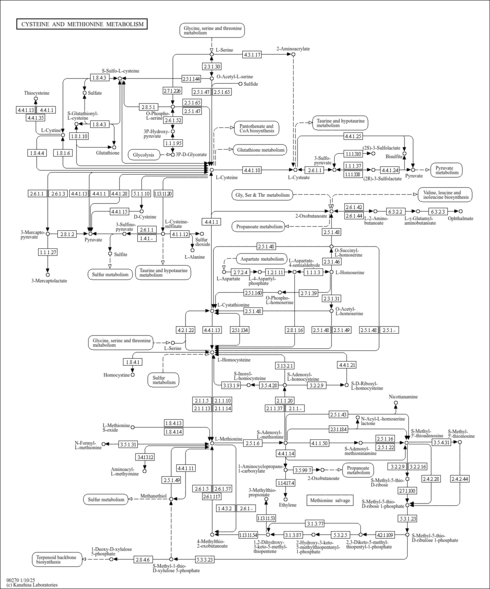
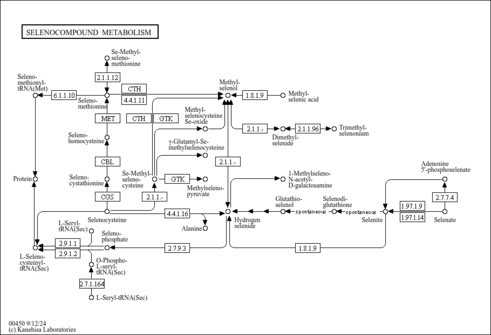
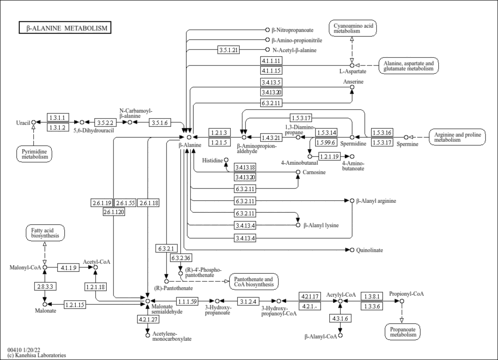
| General Reference | - Thomas, D., Rothstein, R., Rosenberg, N., Surdin-Kerjan, Y. (1988). "SAM2 encodes the second methionine S-adenosyl transferase in Saccharomyces cerevisiae: physiology and regulation of both enzymes." Mol Cell Biol 8:5132-5139.3072475
- Jacq, C., Alt-Morbe, J., Andre, B., Arnold, W., Bahr, A., Ballesta, J. P., Bargues, M., Baron, L., Becker, A., Biteau, N., Blocker, H., Blugeon, C., Boskovic, J., Brandt, P., Bruckner, M., Buitrago, M. J., Coster, F., Delaveau, T., del Rey, F., Dujon, B., Eide, L. G., Garcia-Cantalejo, J. M., Goffeau, A., Gomez-Peris, A., Zaccaria, P., et, a. l. .. (1997). "The nucleotide sequence of Saccharomyces cerevisiae chromosome IV." Nature 387:75-78.9169867
- Garrels, J. I., McLaughlin, C. S., Warner, J. R., Futcher, B., Latter, G. I., Kobayashi, R., Schwender, B., Volpe, T., Anderson, D. S., Mesquita-Fuentes, R., Payne, W. E. (1997). "Proteome studies of Saccharomyces cerevisiae: identification and characterization of abundant proteins." Electrophoresis 18:1347-1360.9298649
- Ghaemmaghami, S., Huh, W. K., Bower, K., Howson, R. W., Belle, A., Dephoure, N., O'Shea, E. K., Weissman, J. S. (2003). "Global analysis of protein expression in yeast." Nature 425:737-741.14562106
- Peng, J., Schwartz, D., Elias, J. E., Thoreen, C. C., Cheng, D., Marsischky, G., Roelofs, J., Finley, D., Gygi, S. P. (2003). "A proteomics approach to understanding protein ubiquitination." Nat Biotechnol 21:921-926.12872131
- Chi, A., Huttenhower, C., Geer, L. Y., Coon, J. J., Syka, J. E., Bai, D. L., Shabanowitz, J., Burke, D. J., Troyanskaya, O. G., Hunt, D. F. (2007). "Analysis of phosphorylation sites on proteins from Saccharomyces cerevisiae by electron transfer dissociation (ETD) mass spectrometry." Proc Natl Acad Sci U S A 104:2193-2198.17287358
|
|---|