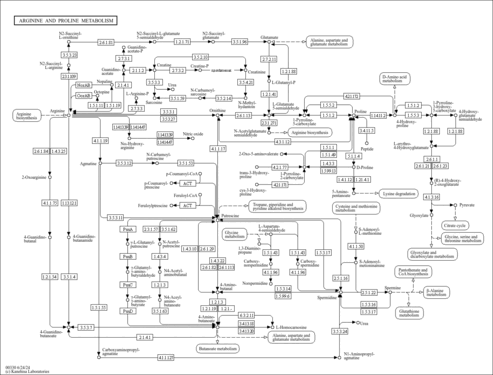
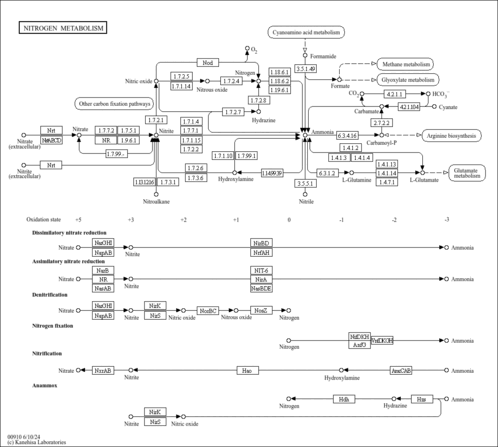
| General Reference | - Minehart, P. L., Magasanik, B. (1992). "Sequence of the GLN1 gene of Saccharomyces cerevisiae: role of the upstream region in regulation of glutamine synthetase expression." J Bacteriol 174:1828-1836.1347768
- Bussey, H., Storms, R. K., Ahmed, A., Albermann, K., Allen, E., Ansorge, W., Araujo, R., Aparicio, A., Barrell, B., Badcock, K., Benes, V., Botstein, D., Bowman, S., Bruckner, M., Carpenter, J., Cherry, J. M., Chung, E., Churcher, C., Coster, F., Davis, K., Davis, R. W., Dietrich, F. S., Delius, H., DiPaolo, T., Hani, J., et, a. l. .. (1997). "The nucleotide sequence of Saccharomyces cerevisiae chromosome XVI." Nature 387:103-105.9169875
- Kim, K. H., Rhee, S. G. (1988). "Sequence of peptides from Saccharomyces cerevisiae glutamine synthetase. N-terminal peptide and ATP-binding domain." J Biol Chem 263:833-838.2891705
- Garrels, J. I., McLaughlin, C. S., Warner, J. R., Futcher, B., Latter, G. I., Kobayashi, R., Schwender, B., Volpe, T., Anderson, D. S., Mesquita-Fuentes, R., Payne, W. E. (1997). "Proteome studies of Saccharomyces cerevisiae: identification and characterization of abundant proteins." Electrophoresis 18:1347-1360.9298649
- Ghaemmaghami, S., Huh, W. K., Bower, K., Howson, R. W., Belle, A., Dephoure, N., O'Shea, E. K., Weissman, J. S. (2003). "Global analysis of protein expression in yeast." Nature 425:737-741.14562106
- Peng, J., Schwartz, D., Elias, J. E., Thoreen, C. C., Cheng, D., Marsischky, G., Roelofs, J., Finley, D., Gygi, S. P. (2003). "A proteomics approach to understanding protein ubiquitination." Nat Biotechnol 21:921-926.12872131
- Hitchcock, A. L., Auld, K., Gygi, S. P., Silver, P. A. (2003). "A subset of membrane-associated proteins is ubiquitinated in response to mutations in the endoplasmic reticulum degradation machinery." Proc Natl Acad Sci U S A 100:12735-12740.14557538
|
|---|