| Identification |
|---|
| Name | 3-hydroxy-3-methylglutaryl-coenzyme A reductase 1 |
|---|
| Synonyms | |
|---|
| Gene Name | HMG1 |
|---|
| Enzyme Class | |
|---|
| Biological Properties |
|---|
| General Function | Involved in hydroxymethylglutaryl-CoA reductase (NADPH) activity |
|---|
| Specific Function | This transmembrane glycoprotein is involved in the control of cholesterol biosynthesis. It is the rate-limiting enzyme of the sterol biosynthesis |
|---|
| Cellular Location | Endoplasmic reticulum membrane; Multi-pass membrane protein |
|---|
| SMPDB Pathways | | Cholesterol biosynthesis and metabolism CE(10:0) | PW002545 | 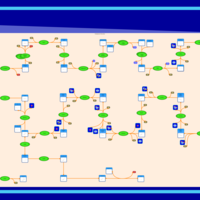   | | Cholesterol biosynthesis and metabolism CE(12:0) | PW002548 | 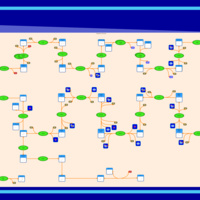 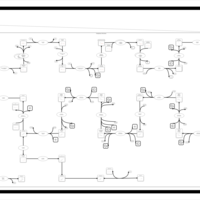 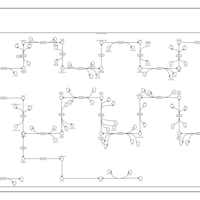 | | Cholesterol biosynthesis and metabolism CE(14:0) | PW002544 |  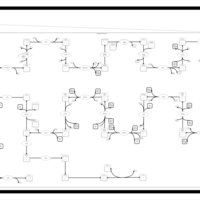 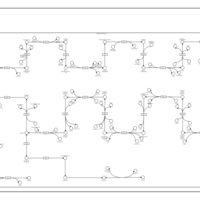 | | Cholesterol biosynthesis and metabolism CE(16:0) | PW002550 | 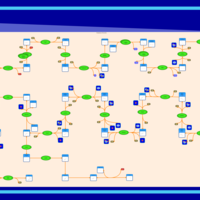 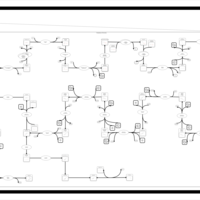 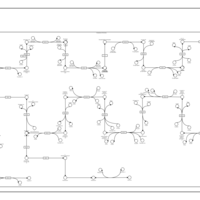 | | Cholesterol biosynthesis and metabolism CE(18:0) | PW002551 |    |
|
|---|
| KEGG Pathways | | Terpenoid backbone biosynthesis | ec00900 | 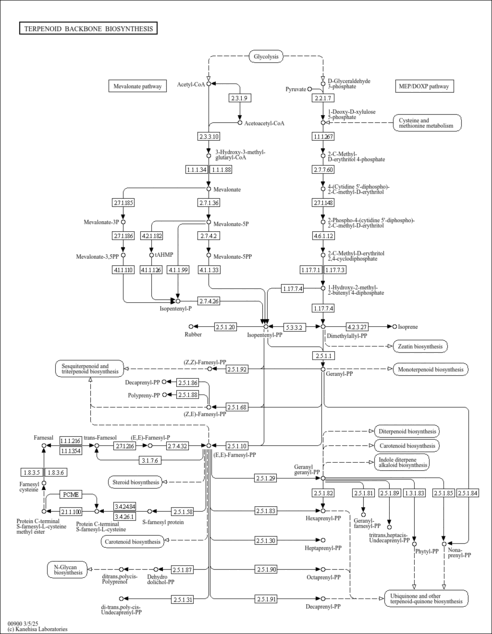 |
|
|---|
| SMPDB Reactions | |
|---|
| KEGG Reactions | |
|---|
| Metabolites | | YMDB ID | Name | View |
|---|
| YMDB00045 | Coenzyme A | Show | | YMDB00310 | 22b-Hydroxycholesterol | Show | | YMDB00426 | NADPH | Show | | YMDB00427 | NADP | Show | | YMDB00707 | (R)-Mevalonic acid | Show | | YMDB00708 | 3-Hydroxy-3-methylglutaryl-CoA | Show | | YMDB00862 | hydron | Show | | YMDB01039 | (R)-Mevalonate | Show |
|
|---|
| GO Classification | | Component |
|---|
| cell part | | membrane part | | intrinsic to membrane | | integral to membrane | | Function |
|---|
| coenzyme binding | | hydroxymethylglutaryl-CoA reductase (NADPH) activity | | binding | | nucleotide binding | | oxidoreductase activity | | cofactor binding | | oxidoreductase activity, acting on CH-OH group of donors | | oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor | | catalytic activity | | NADP or NADPH binding | | Process |
|---|
| lipid metabolic process | | cellular lipid metabolic process | | cellular metabolic process | | isoprenoid metabolic process | | isoprenoid biosynthetic process | | primary metabolic process | | coenzyme A metabolic process | | oxidation reduction | | cofactor metabolic process | | coenzyme metabolic process | | metabolic process |
|
|---|
| Gene Properties |
|---|
| Chromosome Location | chromosome 13 |
|---|
| Locus | YML075C |
|---|
| Gene Sequence | >3165 bp
ATGCCGCCGCTATTCAAGGGACTGAAACAGATGGCAAAGCCAATTGCCTATGTTTCAAGA
TTTTCGGCGAAACGACCAATTCATATAATACTTTTTTCTCTAATCATATCCGCATTCGCT
TATCTATCCGTCATTCAGTATTACTTCAATGGTTGGCAACTAGATTCAAATAGTGTTTTT
GAAACTGCTCCAAATAAAGACTCCAACACTCTATTTCAAGAATGTTCCCATTACTACAGA
GATTCCTCTCTAGATGGTTGGGTATCAATCACCGCGCATGAAGCTAGTGAGTTACCAGCC
CCACACCATTACTATCTATTAAACCTGAACTTCAATAGTCCTAATGAAACTGACTCCATT
CCAGAACTAGCTAACACGGTTTTTGAGAAAGATAATACAAAATATATTCTGCAAGAAGAT
CTCAGTGTTTCCAAAGAAATTTCTTCTACTGATGGAACGAAATGGAGGTTAAGAAGTGAC
AGAAAAAGTCTTTTCGACGTAAAGACGTTAGCATATTCTCTCTACGATGTATTTTCAGAA
AATGTAACCCAAGCAGACCCGTTTGACGTCCTTATTATGGTTACTGCCTACCTAATGATG
TTCTACACCATATTCGGCCTCTTCAATGACATGAGGAAGACCGGGTCAAATTTTTGGTTG
AGCGCCTCTACAGTGGTCAATTCTGCATCATCACTTTTCTTAGCATTGTATGTCACCCAA
TGTATTCTAGGCAAAGAAGTTTCCGCATTAACTCTTTTTGAAGGTTTGCCTTTCATTGTA
GTTGTTGTTGGTTTCAAGCACAAAATCAAGATTGCCCAGTATGCCCTGGAGAAATTTGAA
AGAGTCGGTTTATCTAAAAGGATTACTACCGATGAAATCGTTTTTGAATCCGTGAGCGAA
GAGGGTGGTCGTTTGATTCAAGACCATTTGCTTTGTATTTTTGCCTTTATCGGATGCTCT
ATGTATGCTCACCAATTGAAGACTTTGACAAACTTCTGCATATTATCAGCATTTATCCTA
ATTTTTGAATTGATTTTAACTCCTACATTTTATTCTGCTATCTTAGCGCTTAGACTGGAA
ATGAATGTTATCCACAGATCTACTATTATCAAGCAAACATTAGAAGAAGACGGTGTTGTT
CCATCTACAGCAAGAATCATTTCTAAAGCAGAAAAGAAATCCGTATCTTCTTTCTTAAAT
CTCAGTGTGGTTGTCATTATCATGAAACTCTCTGTCATACTGTTGTTTGTCTTCATCAAC
TTTTATAACTTTGGTGCAAATTGGGTCAATGATGCCTTCAATTCATTGTACTTCGATAAG
GAACGTGTTTCTCTACCAGATTTTATTACCTCGAATGCCTCTGAAAACTTTAAAGAGCAA
GCTATTGTTAGTGTCACCCCATTATTATATTACAAACCCATTAAGTCCTACCAACGCATT
GAGGATATGGTTCTTCTATTGCTTCGTAATGTCAGTGTTGCCATTCGTGATAGGTTCGTC
AGTAAATTAGTTCTTTCCGCCTTAGTATGCAGTGCTGTCATCAATGTGTATTTATTGAAT
GCTGCTAGAATTCATACCAGTTATACTGCAGACCAATTGGTGAAAACTGAAGTCACCAAG
AAGTCTTTTACTGCTCCTGTACAAAAGGCTTCTACACCAGTTTTAACCAATAAAACAGTC
ATTTCTGGATCGAAAGTCAAAAGTTTATCATCTGCGCAATCGAGCTCATCAGGACCTTCA
TCATCTAGTGAGGAAGATGATTCCCGCGATATTGAAAGCTTGGATAAGAAAATACGTCCT
TTAGAAGAATTAGAAGCATTATTAAGTAGTGGAAATACAAAACAATTGAAGAACAAAGAG
GTCGCTGCCTTGGTTATTCACGGTAAGTTACCTTTGTACGCTTTGGAGAAAAAATTAGGT
GATACTACGAGAGCGGTTGCGGTACGTAGGAAGGCTCTTTCAATTTTGGCAGAAGCTCCT
GTATTAGCATCTGATCGTTTACCATATAAAAATTATGACTACGACCGCGTATTTGGCGCT
TGTTGTGAAAATGTTATAGGTTACATGCCTTTGCCCGTTGGTGTTATAGGCCCCTTGGTT
ATCGATGGTACATCTTATCATATACCAATGGCAACTACAGAGGGTTGTTTGGTAGCTTCT
GCCATGCGTGGCTGTAAGGCAATCAATGCTGGCGGTGGTGCAACAACTGTTTTAACTAAG
GATGGTATGACAAGAGGCCCAGTAGTCCGTTTCCCAACTTTGAAAAGATCTGGTGCCTGT
AAGATATGGTTAGACTCAGAAGAGGGACAAAACGCAATTAAAAAAGCTTTTAACTCTACA
TCAAGATTTGCACGTCTGCAACATATTCAAACTTGTCTAGCAGGAGATTTACTCTTCATG
AGATTTAGAACAACTACTGGTGACGCAATGGGTATGAATATGATTTCTAAAGGTGTCGAA
TACTCATTAAAGCAAATGGTAGAAGAGTATGGCTGGGAAGATATGGAGGTTGTCTCCGTT
TCTGGTAACTACTGTACCGACAAAAAACCAGCTGCCATCAACTGGATCGAAGGTCGTGGT
AAGAGTGTCGTCGCAGAAGCTACTATTCCTGGTGATGTTGTCAGAAAAGTGTTAAAAAGT
GATGTTTCCGCATTGGTTGAGTTGAACATTGCTAAGAATTTGGTTGGATCTGCAATGGCT
GGGTCTGTTGGTGGATTTAACGCACATGCAGCTAATTTAGTGACAGCTGTTTTCTTGGCA
TTAGGACAAGATCCTGCACAAAATGTTGAAAGTTCCAACTGTATAACATTGATGAAAGAA
GTGGACGGTGATTTGAGAATTTCCGTATCCATGCCATCCATCGAAGTAGGTACCATCGGT
GGTGGTACTGTTCTAGAACCACAAGGTGCCATGTTGGACTTATTAGGTGTAAGAGGCCCG
CATGCTACCGCTCCTGGTACCAACGCACGTCAATTAGCAAGAATAGTTGCCTGTGCCGTC
TTGGCAGGTGAATTATCCTTATGTGCTGCCCTAGCAGCCGGCCATTTGGTTCAAAGTCAT
ATGACCCACAACAGGAAACCTGCTGAACCAACAAAACCTAACAATTTGGACGCCACTGAT
ATAAATCGTTTGAAAGATGGGTCCGTCACCTGCATTAAATCCTAA |
|---|
| Protein Properties |
|---|
| Pfam Domain Function | |
|---|
| Protein Residues | 1054 |
|---|
| Protein Molecular Weight | 115624.0 |
|---|
| Protein Theoretical pI | 8.09 |
|---|
| Signalling Regions | |
|---|
| Transmembrane Regions | - 27-53
- 187-211
- 242-266
- 300-324
- 332-357
- 398-422
- 499-524
|
|---|
| Protein Sequence | >3-hydroxy-3-methylglutaryl-coenzyme A reductase 1
MPPLFKGLKQMAKPIAYVSRFSAKRPIHIILFSLIISAFAYLSVIQYYFNGWQLDSNSVF
ETAPNKDSNTLFQECSHYYRDSSLDGWVSITAHEASELPAPHHYYLLNLNFNSPNETDSI
PELANTVFEKDNTKYILQEDLSVSKEISSTDGTKWRLRSDRKSLFDVKTLAYSLYDVFSE
NVTQADPFDVLIMVTAYLMMFYTIFGLFNDMRKTGSNFWLSASTVVNSASSLFLALYVTQ
CILGKEVSALTLFEGLPFIVVVVGFKHKIKIAQYALEKFERVGLSKRITTDEIVFESVSE
EGGRLIQDHLLCIFAFIGCSMYAHQLKTLTNFCILSAFILIFELILTPTFYSAILALRLE
MNVIHRSTIIKQTLEEDGVVPSTARIISKAEKKSVSSFLNLSVVVIIMKLSVILLFVFIN
FYNFGANWVNDAFNSLYFDKERVSLPDFITSNASENFKEQAIVSVTPLLYYKPIKSYQRI
EDMVLLLLRNVSVAIRDRFVSKLVLSALVCSAVINVYLLNAARIHTSYTADQLVKTEVTK
KSFTAPVQKASTPVLTNKTVISGSKVKSLSSAQSSSSGPSSSSEEDDSRDIESLDKKIRP
LEELEALLSSGNTKQLKNKEVAALVIHGKLPLYALEKKLGDTTRAVAVRRKALSILAEAP
VLASDRLPYKNYDYDRVFGACCENVIGYMPLPVGVIGPLVIDGTSYHIPMATTEGCLVAS
AMRGCKAINAGGGATTVLTKDGMTRGPVVRFPTLKRSGACKIWLDSEEGQNAIKKAFNST
SRFARLQHIQTCLAGDLLFMRFRTTTGDAMGMNMISKGVEYSLKQMVEEYGWEDMEVVSV
SGNYCTDKKPAAINWIEGRGKSVVAEATIPGDVVRKVLKSDVSALVELNIAKNLVGSAMA
GSVGGFNAHAANLVTAVFLALGQDPAQNVESSNCITLMKEVDGDLRISVSMPSIEVGTIG
GGTVLEPQGAMLDLLGVRGPHATAPGTNARQLARIVACAVLAGELSLCAALAAGHLVQSH
MTHNRKPAEPTKPNNLDATDINRLKDGSVTCIKS |
|---|
| References |
|---|
| External Links | |
|---|
| General Reference | - Basson, M. E., Thorsness, M., Finer-Moore, J., Stroud, R. M., Rine, J. (1988). "Structural and functional conservation between yeast and human 3-hydroxy-3-methylglutaryl coenzyme A reductases, the rate-limiting enzyme of sterol biosynthesis." Mol Cell Biol 8:3797-3808.3065625
- Bowman, S., Churcher, C., Badcock, K., Brown, D., Chillingworth, T., Connor, R., Dedman, K., Devlin, K., Gentles, S., Hamlin, N., Hunt, S., Jagels, K., Lye, G., Moule, S., Odell, C., Pearson, D., Rajandream, M., Rice, P., Skelton, J., Walsh, S., Whitehead, S., Barrell, B. (1997). "The nucleotide sequence of Saccharomyces cerevisiae chromosome XIII." Nature 387:90-93.9169872
- Basson, M. E., Thorsness, M., Rine, J. (1986). "Saccharomyces cerevisiae contains two functional genes encoding 3-hydroxy-3-methylglutaryl-coenzyme A reductase." Proc Natl Acad Sci U S A 83:5563-5567.3526336
- Li, X., Gerber, S. A., Rudner, A. D., Beausoleil, S. A., Haas, W., Villen, J., Elias, J. E., Gygi, S. P. (2007). "Large-scale phosphorylation analysis of alpha-factor-arrested Saccharomyces cerevisiae." J Proteome Res 6:1190-1197.17330950
- Smolka, M. B., Albuquerque, C. P., Chen, S. H., Zhou, H. (2007). "Proteome-wide identification of in vivo targets of DNA damage checkpoint kinases." Proc Natl Acad Sci U S A 104:10364-10369.17563356
- Albuquerque, C. P., Smolka, M. B., Payne, S. H., Bafna, V., Eng, J., Zhou, H. (2008). "A multidimensional chromatography technology for in-depth phosphoproteome analysis." Mol Cell Proteomics 7:1389-1396.18407956
|
|---|
